|
Size: 41819
Comment:
|
Size: 42441
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 429: | Line 429: |
| Although the choice of the optimal parcellation scheme for the source space is not easy. The optimal choice is to choose a parcellation based on anatomy, for example the Brodmann parcellation [[http://www.nature.com/nature/journal/v536/n7615/full/nature18933.html|here]]. In brainstorm these atlases are imported in Brainstorm as scouts (cortical regions of interest), and saved directly in the surface files. [[https://neuroimage.usc.edu/brainstorm/Tutorials/LabelFreeSurfer?highlight=(parcellation)#Cortical_parcellations|https://neuroimage.usc.edu/brainstorm/Tutorials/LabelFreeSurfer?highlight=%28parcellation%29#Cortical_parcellations]] In this tutorial, we will use the scouts " Destrieux atlas'''" (following figure)''' The following matrix is the solution that we obtain with these scouts |
Although the choice of the optimal parcellation scheme for the source space is not easy. The optimal choice is to choose a parcellation based on anatomy, for example the Brodmann parcellation [[http://www.nature.com/nature/journal/v536/n7615/full/nature18933.html|here]]. In brainstorm these atlases are imported in Brainstorm as scouts (cortical regions of interest), and saved directly in the surface files as explained in this tutorial [[https://neuroimage.usc.edu/brainstorm/Tutorials/LabelFreeSurfer?highlight=(parcellation)#Cortical_parcellations|here]]. In this tutorial, we will use the scouts " Destrieux atlas'''" (following figure)''' {{attachment:destrieuxScouts.png||height="400",width="500"}} To select this atlas, from the connectivity menu, you have to check the box 'use scouts', select the scout function 'mean' and apply the function 'Before', save individual results. In this tutorial, we select the correlation as example, the same process is expected for the other methods. {{attachment:processConnectivityScouts.png||height="400",width="300"}} For more detail for these options please refer to this [[https://neuroimage.usc.edu/forums/t/choosing-scout-function-before-or-after/2454|thread]] The following matrix is the solution that we obtain with these scouts with the size of 148x148 for this atlas (~400 Ko) {{attachment:connectivityScoutDistrieux.png||height="400",width="500"}} |
Tutorial 28: Connectivity
[TUTORIAL UNDER DEVELOPMENT: NOT READY FOR PUBLIC USE]
Authors: Hossein Shahabi, Mansoureh Fahimi, Francois Tadel, Esther Florin, Sergul Aydore, Syed Ashrafulla, Takfarinas Medani, Elizabeth Bock, Sylvain Baillet
Contents
- Introduction
- General terms/considerations for a connectivity analysis
- Simulated data (AR model)
- Coherence (FFT-based)
- Granger Causality
- Coherence and envelope (Hilbert/Morlet)
- Simulated data (phase synchrony)
- Correlation
- Phase locking value
- Method selection and comparison
- Connectivity measures on real data
- Phase Locking Value (PLV)
- Coherence (COH)
- Correlation (COR)
- Spectral Granger Causality (SGC)
- Phase Transfer Entropy (PTE)
- TODO : Connectivity measure on MEG data
- TODO : Electrodes level
- TODO : Sources level
- Granger Causality - Mathematical Background
- Additional documentation
Introduction
During the past few years, the research focus in brain imaging moved from localizing functional regions to understanding how different regions interact together. It is now widely accepted that some of the brain functions are not supported by isolated regions but rather by a dense network of nodes interacting in various ways.
Brain networks (connectivity) is a recently developed field of neuroscience which investigates interactions among regions of this vital organ. These networks can be identified using a wide range of connectivity measures applied on neurophysiological signals, either in time or frequency domain. The knowledge provides a comprehensive view of brain functions and mechanisms.
This module of Brainstorm tries to facilitate the computation of brain networks and the representation of their corresponding graphs. Figure 1 illustrates a general framework to analyze brain networks. Preprocessing and source localization tasks for neural data are thoroughly described in previous sections of this tutorial. The connectivity module is designed to carry out remained steps, including the computation of connectivity measures, and statistical analysis and visualizations of networks.
General terms/considerations for a connectivity analysis
Sensors vs sources: The connectivity analysis can be performed either on sensor data (like EEG, MEG signals) or reconstructed sources (voxels/scouts).
Nature of the signals: The selection of connectivity method depends on the nature of the data. Some approaches are more suitable for spontaneous data (resting state) while others work better with task data (trials).
Point-based connectivity vs. full network: Most of the connectivity functions in this toolbox have the option to either compute the connectivity between one point (channel) and the rest of the network (1 x N) or the entire network (N x N). While the later calculates the graph thoroughly, the first option enjoys a faster computation and it is more useful when you are interested in the connectivity of an ROI with the other regions of the brain.
Temporal resolution: Connectivity networks can be computed in two ways; static and dynamic. Time-varying networks can present the dynamics of brain networks. In contrast, the static graphs illustrate a general perspective of brain connectivity which is helpful in specific conditions. Users need to decide which type of network is more informative for their study.
Time-frequency transformation: Many connectivity measures use a time-frequency transformation to transfer the data to the frequency domain. These approaches include short time Fourier transform, Hilbert transform, and Morlet wavelet.
Output data structure: Consequently, computed connectivity matrices in this toolbox have up to four dimensions; channels x channels x frequency bands x time. Also, when dealing with trials and several files, each file has that 4-D structure.
Simulated data (AR model)
In order to compare different connectivity measures, we use simulated data with known ground truth. Three channels are

where a1,...,an are coefficients of an all-pass filter and b1,...,bn are coefficients for a lowpass filter which describes the transfer function from channel 2 to channel 3. One can take the Fourier trnasform from these equations and rearrange them in a matrix form. Based on this model, we generated signals and tested the available connectivity measures. This dataset is available here.
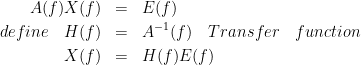
TODO : explain the figure
Coherence (FFT-based)
The coherence is a statistical measure which computes the relation between two signals, like x(t) and y(t), in the frequency domain. The magnitiude-squared coherence is,

The coherence function uses the Fourier transform to compute the spectral densities. A related measure which alleviates the problem of volume conduction is the lagged-coherence (ref).
![\begin{eqnarray*}
LC_{xy}(f) = \frac{Im \left (S_{xy}(f) \right )}{\sqrt{ S_{xx}(f)S_{yy}(f) - \left [ Re\left ( S_{xy}(f) \right ) \right ]^{2} }} \\
\end{eqnarray*} \begin{eqnarray*}
LC_{xy}(f) = \frac{Im \left (S_{xy}(f) \right )}{\sqrt{ S_{xx}(f)S_{yy}(f) - \left [ Re\left ( S_{xy}(f) \right ) \right ]^{2} }} \\
\end{eqnarray*}](/brainstorm/Tutorials/Connectivity?action=AttachFile&do=get&target=latex_800c7c6d6923f7c1a323462a6746f1ec8dbdca42_p1.png)
TODO : explain the elememts of the equation
- Put the Simulated data in the Process1 tab.
- Click on [Run] to open the Pipeline editor.
Run the process: Connectivity > Coherence NxN
- Set the options as follows:
Time window: Select the entire signal.
Removing evoked response: Check this box to remove the averaged evoked response from the individual trials.
Measure: You can select either the "Magnitude-Squared" coherence or the "Imaginary" coherence. we first select the former one.
Maximum frequency resolution: This value characterizes the distance between frequency bins. Smaller values give higher resolutions but probably noisier.
Highest frequency of interest: It specifies the highest frequency which should be analyzed. Here, we selected Fs/2 = 125 Hz to have the coherence in all frequencies.
Output configuration: Select one file per input file.
In general, after running the connectivity processes, you can find a multi-dimensional matrix of connectivity in the database. In order to represent this matrix, there are several options.
Right click on the file and select Power spectrum and Display as image These two figures are plotted here. The left figure shows the Coherence values in all analyzed frequencies. Another figure displays the Transfer matrix between channels for the selected frequency bin.
Similarly, we can run this process and select "imaginary coherence". which gives us the following representation,
Granger Causality
Granger Causality (GC) is a method of functional connectivity, adapted by Clive Granger in the 1960s, but later refined by John Geweke in the form that is used today. Granger Causality is originally formulated in economics but has caught the attention of the neuroscience community in recent years. Before this, neuroscience traditionally relied on stimulation or lesioning a part of the nervous system to study its effect on another part. However, Granger Causality made it possible to estimate the statistical influence without requiring direct intervention (ref: wiener-granger causality a well-established methodology).
Granger Causality is a measure of linear dependence, which tests whether the variance of error for a linear autoregressive model estimation of a signal (A) can be reduced when adding a linear model estimation of a second signal (B). If this is true, signal B has a Granger Causal effect on the first signal A, i.e., independent information of the past of B improves the prediction of A above and beyond the information contained in the past of A alone. The term independent is emphasized because it creates some interesting properties for GC, such as that it is invariant under rescaling of A and B, as well as the addition of a multiple of A to B. The measure of Granger Causality is nonnegative, and zero when there is no Granger causality(Geweke, 1982).
The main advantage of Granger Causality is that it is an asymmetrical measure, in that it can dissociate between A->B versus B->A. It is important to note however that though the directionality of Granger Causality is a step closer towards measuring effective connectivity compared to symmetrical measures, it should still not be confused with “true causality”. Effective connectivity estimates the effective mechanism generating the observed data (model-based approach), whereas GC is a measure of causal effect based on prediction, i.e., how well the model is improved when taking variables into account that are interacting (data-driven approach) (Barrett and Barnett, 2013). The difference with causality is best illustrated when there are more variables interacting in a system than those considered in the model. For example, if a variable C is causing both A and B, but with a smaller delay for B than for A, then a GC measure between A and B would show a non-zero GC for B->A, even though B is not truly causing A (Bressler and Seth, 2011).
The complete formulation of this method is discussed in the advanced section. Here, we apply the Granger causality and its spectral version on the simulated data.
- Put the Simulated data in the Process1 tab.
- Click on [Run] to open the Pipeline editor.
Run the process: Connectivity > Bivariate Granger causality NxN
Input options:
Time window: specifies the time window you want to use for your model.
Remove evoked response from each trial: this option refers to subtracting the average of phase-locked activity (ERP) from each individual trial. Presently some studies measure interdependency of ongoing brain activity by removing the average event-related potential from each trial. It is also recommended by some as it meets the zero-mean stationarity requirement (improves stationarity of the system). However, the problem with this approach is that it does not account for trial-to-trial variability (For a discussion see (Wang et al., 2008)).
Estimator options:
Model order: Selection of model order is a critical issue and is typically evaluated from criteria derived from information theory. Several criteria have been proposed, of which the most used are Akaike’s information criterion, the Bayesian-Schwartz’s criterion, and the Hannan-Quinn criterion (Koichi and Antonio, 2014). Model fitting quality crucially depends on the proper model order selection. Too low orders may lack the necessary details, while too big orders tend to create spurious values of connectivity. Note that in our simulated example even though the simulation was created with an underlying model of 4, a Granger model order of 6 was selected with decent resulting connectivity.
Output options:
Save individual results (one file per input file): option to save GC estimates on several files separately.
Concatenate input files before processing (one file): option to save GC estimates on several files as one concatenated matrix.
Spectral Granger causality
Coherence and envelope (Hilbert/Morlet)
This process
- Put the Simulated data in the Process1 tab.
- Click on [Run] to open the Pipeline editor.
Run the process: Connectivity > HCorr NxN
Input Options: The time range of the input signal can be specified here. Also, bad channels and the evoked response of trials can be discarded, if appropriate.
Time-frequency transformation method: The method for this transformation (Hilbert transform or Morlet Wavelet) should be selected. Additionally, this analysis needs further inputs, e.g. frequency ranges, number of bins, and Morlet parameters, which can be defined by an external panel as depicted in Figure 4 (By clicking on “Edit”). A complete description regarding time-frequency transformation can be found here. In the context of connectivity study, we must analyze complex output values of these functions, so two other options (power and magnitude) are disabled on the bottom of this panel.
Signal splitting: This process has the capability of splitting the input data into several blocks for performing time-frequency transformation, and then merging them to build a single file. This feature helps to save a huge amount of memory and, at the same time, avoid breaking a long-time recording to short-time signals, which makes inconsistency in dynamic network representation of spontaneous data. The maximum number of blocks which can be specified is 20.
Connectivity measure: Here, three major and widely used coherence based measures of brain connectivity can be computed. Next, desired parameters for windowing, i.e. window length and overlap, should be determined. Please note that these values are usually defined based on the nature of data, the purpose of the study, and the selected connectivity measure.
Parallel processing: This feature, which is only applicable for envelope correlation, employs the parallel processing toolbox in Matlab to fasten the computational procedure. As described in the advanced section of this tutorial, envelope correlation utilizes a pairwise orthogonalization approach to attenuate the cross-talk between signals. This process requires heavy computation, especially for a large number of channels, however, using Parallel Processing Toolbox, the software distributes calculations on several threats of CPU. The maximum number of pools varies on each computer and it is dependent on the CPU.
Output configuration: Generally, the above calculation results in a 4-D matrix, where dimensions represent channels (1st and 2nd dimensions), time points (3rd dimension), and frequency (4th dimension). In the case that we analyze event-related data, we have also several files (trials). However, due to poor signal to noise ratio of a single trial, an individual realization of connectivity matrices for each of them is not in our interests. Consequently, we need to average connectivity matrices among all trials of a specific event. The second option of this part performs this averaging.
Simulated data (phase synchrony)
Correlation
The correlation is the basic approach to show the dependence or association among two random variables or MEG/EEG signals. While this method has been widely used in electrophysiology, it should not be considered as the best technique for finding the connectivity matrices. The correlation by its nature fails to alleviate the problem of volume conduction and cannot explain the association in different frequency bands. However, it still can provide valuable information in case we deal with a few narrow-banded signals.
- Put the Simulated data in the Process1 tab.
- Click on [Run] to open the Pipeline editor.
Run the process: Connectivity > Correlation NxN
- Set the options as follows:
Time window: Select the entire signal.
Estimator options: leave the box unchecked so the means will be subtracted before computing the correlation.
Output configuration: Select one file per input.
Phase locking value
Method selection and comparison
We can have a comparison between different connectivity functions. The following table briefly does this job.
Comparing different approaches with the ground truth we find out that the HCorr function works slightly better than other coherence functions.
Connectivity measures on real data
In this section we will show how to use the Brainstorm connectivity tools on real data.
LFP data recorded on monkey
Experimental Setup and data recording:
For this part we will use the LFP monkey data described in Bresslers et al (1993), these data are widely used over the last past years in many studies.
The original data could be found in this link more information on the data organization is explained here and also here
These recordings were made using 51um diameter bipolar electrodes, separated by 2.5mm in macaque monkeys. For our analysis, we have selected the monkey named GE.
The signals are recorded from a total of 15 electrode pairs in the right hemisphere. The data are recorded from 6 main areas of the right cortex (Straite, Prestriate, Parietal, Somato, Motor, and frontal cortex). The locations of the 15 electrodes are shown in this figure. Voltages are recorded at 200Hz sampling rate.
On the left, a scheme of the monkey brain area, on the right, the locations of 15 electrode pairs in the right hemisphere (reproduced from Aydore et al. (2013) and Bresslers et al (1993).)
In these experiments, the monkey was trained to depress a lever and wait for a visual stimulus that informs the monkey to either let go the lever (release/GO) or keep the lever down (not release/NOGO). The visual stimulus is presented with four dots on a screen. The dots form either a shape of a diagonal line or a shape of a diamond.
For our analysis in this tutorial, we select a dataset with a diagonal line as the 'NOGO' stimulus and the diamond as the 'GO'.
For more details about the experiment please refer to Bresslers et al (1993).
Importing and analyzing data within brainstorm:
For our case, we imported and adapted the data to the brainstorm format, you can download a sample of the data here. (this sample contains only 50 epochs per condition, the full data should uploaded asap)
Voltage is in uV and was recorded at 200Hz sampling rate. After pooling and ordering the dataset together, we select 480 trials for each condition (GO and NOGO).
Defining the lever initial descent to be at time t = 0ms. Each trial lasts 600ms, the stimulus was given 100ms after the lever was depressed, and last for 100ms. On go trials, a water reward was provided 500 ms after stimulus onset if the hand was lifted within the 500ms. On the nogo trials, the lever was depressed for 500ms.
In the following figure, the time line of the averaged response for the 480 epochs of the GO condition. The blue is at t = 0ms begening of the recording, the green line is the stimulus onset at t = 100ms, the orange line is the mean time of the response onset in the case of the GO condition (release the lever), the red line is the time cursor, set at t = 250ms, time that we choose to separate between early response and late response in this tutorial.
In this analysis, we will focus mainly on two windows. The early answer for t ϵ[100, 250 ms] in which we expect a visual activation on the occipital area (striate and prestriate areas, group of electrodes 1 to 6).
We will also show some analysis on the late answer, for t ϵ [250, 450 ms] in which we expect an activation/connectivity between the striate and motor cortex in the GO condition and no or less activation for the NOGO condition.
Process of computation :
For all the connectivity measures, we will compute the the matrix (NxN) for all the trials and for the both conditions (GO snd NOGO), then we will average the measure for each condition. Afterward we will analyse the average matrix between the condition.
Step 1 : Drag and drop all the trials for both condition in Process1 tab.
Step 2 : Click on [Run] to open the Pipeline editor. Select the desired connectivity measure, choose the desired parameters (see bellow) and click on Run the computation.
- After running the connectivity processes, you can find a multi-dimensional matrix of connectivity in the database. This matrix is computed for each trial. But in this case s=we need to average all these data.
Step 3 : Average the connectivity measure for all trial bellonging the same condition.
On the Process1, select the option 'time freq' process, and click on Run. Select the process "Average > Average files". Select the options: By trial group (folder), Arithmetic average, Keep all the event markers, then lunch to computation.
- An average of the connectivity measure is computed and appeares in the Brainstorm database window.
The only file we need is the averaged connectivity for each condition. To free space in your hard disc and in order to be able to compute other connectivity meaure, you can/should remove the previous individual connectivity for each trial. To do so, keep the 'time freq' in process1, click Run and choose : File>Delete File>Delete selected Files and then click on run.
Phase Locking Value (PLV)
We select the two groupes of files in the the process 1, then hit Run, select Connectivity then Phase Locking Value NxN. Choose time window between 100 to 250ms (for early analysis and 250 to 450 ms for the late answer).
For the frequency bands for Hilbert transform, we select narrow bands from 12 to 60 Hz with bandwidh of 10Hz. The PLV is more accurate on short frequency band and to pretend for significant value we recommadn to use windows with more than 100 samples.
For the remaining, keep the other options as they are, select the Magnitude and choose the option 'Save individual results (one file per input file)' and finaly click on Run.
This could take some times according to your computer and the size of your data.
Now, in order to have one measure for each condition, we need to average all the connectivity measure across trials. We do this from the Brainstorm Process1 window, select 'Process time-freq' (the third icone). Then click on run and select : Average->Average Files, select the option : By trial group (folder average) with the function Arithmetic average. This will compute the average connectivity PLV matrix for the Go and NoGo stimulus.
In order to represent this matrix, there are several options.
Right click on the connectivity file and select the first option > Display as graph [NxN]. We display both figures for the GO (left figure) and NOGO (right figure) conditions.
As explained above, we will focuse on the early response in which we expect high connectivity measures on the striate and prestriate areas. For the late answer, we expect high measures on/between the occipital and motor cortex du to the eventual hand movements..
Early answer t ϵ [100-250 ms]:
From the brainstrom control panel "Display", we can tune the value of the freauency band from the cursor. Same options are available for the connectivity threshold and for the distance filtring. You can play with the connectivity threshold in order to show more or less connectivity between the nodes. The distance filtreing is also important to set in order to set a limit distance between two nodes (electrodes in this example).
Setting : distance filtering 0mm, band1, threshold 0.844.
For these dta we don't have he exact location of the electrode on the cortex, we buil an approximation of the location, therefore we will not set the distance filtring to zero in this case.
As we expected, the results show high connectivity value (PLV) between the striate and prestriate area. The strength of the measures is almost the same for both conditions, however, we observe some difference on the electrodes between the Line and the Diamond, this is probably due to the difference on the shape (patern) of the visual stimuli (?https://www.physiology.org/doi/pdf/10.1152/jn.1996.75.1.481).
We will also display the connectivity matrix as an image, either by selecting the measure and press 'Enter' or Right-click on the connectivity file and select the first option > Display as an image [NxN]
As we saw before, the highest value are between the electrodes 2, 3, 4, 5, and 6 which are in the occipital cortex.
To haghlight the difference between the two condition, we can use the Process2 and compute the difference between the two images. (Further methods for statistics are under devellopoment)
From the Process2 bar, we can compute the difference between the images in order to highlight the main differencs between the two condition. To do that, you jus drag the qssociqted connectivity file for the condition one to the Files A and the condition two to the files B, then click on run, select Difference, then one of the propsed option, in this tutorial we selected the 'Normalized : A-B/A+B'.
The resulted image shows the highest difference in the connectivity is between the electrodes (2-6) and (3-6), this is exactely the difference that we observed in the previous graphes, which is mainly the difference between the two conditions.
Late answer t ϵ [250-450 ms] :
distance filtering : 0mm, band1, threshold 0.644
In this late answer, also as we expected, we see higher value in the GO condition then the NOGO condition. Also we observe a connexion between the striate to motot cortex, this connection is related to the mouvmenet of the hand to release the lever.
These results show also the connection between the striat/prestiriate to the frontal regions, since this later is involved in the selection of actions based on perceptual cues and reward values (?https://www.sciencedirect.com/science/article/pii/S2352154614000278.)
The frontal regions are involved in the selection of actions based on perceptual cue and reward values. (?https://reader.elsevier.com/reader/sd/pii/S2352154614000278?token=E7ADF055DE559B634D31E487327596C22197ACFAB9CD133691DE4AC1B883705C1B8A4DA41C3214948767BA70451906C0)
These connectivity results are highly correlated to the ones observed witin the previous publications Aydore et al. (2013) and Bresslers et al (1993). We should also notify that is in this process, the difference in the results is related to the implementation, the selected time windows and the sample data(here we picked randomly 480 samples from each condition).
As in previous, we will visualize the results as an image for the difference between the two conditions.
Of course, ultimately statistical analysis of these maps is required to make scientific inferences from your data.
From this matrix, we can check pixels by pixels the differents value abd combinaison of electrodes, there is difference between the electrodes (1,4), (2,4), (1,8), (2,9), (2,10), (4,8), (7,8) but also it show highest value between (3,9) and (9,14).
If we choose the option that remove the ERP from each trial before computing the connectivity, we end up with these results.
This brings the signals to a slightly more stationary state [refer to the explanation in the bottom]
Results without ERP (function to remove the erp is not available on bst for plv for now) :
Early answer t ϵ [100-250 ms] :
distance filtering : 0mm, band1, threshold 0.75
Late answer t ϵ [250-450 ms] :
distance filtering : 0mm, band1, threshold 0.666
Coherence (COH)
We use the same data as previous, and we will compute the coherence. We will try to show similar results as shown in the Bresslers et al in which high value of coherence are observed between the striate and motor cortex areas within the freauency band 12-25 Hz.
Early answer :
Results with ERP : distance filtering : 0mm, band1, threshold 0.5
Late answer :
Results with ERP : distance filtering : 0mm, band1, threshold 0.667
Results without the ERP :
Early answer : distance filtering : 0mm, band1, threshold 0.5
same figure for both condition ///
Late answer : distance filtering : 0mm, band1, threshold 0.667
Correlation (COR)
We use the same data as previous, and we will compute the correlation. As explained before, the correlation is the basic and simple method to observe interaction between region. We will try to show similar results as shown in the Bresslers et al.
Late answer t ϵ [250-450 ms] :
Results with ERP : distance filtering : 0mm, threshold 0.7
Results without ERP: distance filtering : 0mm, threshold 0.63
Spectral Granger Causality (SGC)
Early answer: with ERP
- distance filtering : 0mm, threshold 2.5, band1 12.5
late answer: with erp
- distance filtering: 0mm, threshold 0.315, band1 12.5
Early answer: without ERP
- distance filtering : 0mm, threshold ~0.5, band1 12.5
Late answer: without ERP
- distance filtering : 0mm, threshold ~0.27, band1 12.5
Phase Transfer Entropy (PTE)
early with erp 0,1, b1. 0mm ...not relevant > recheck
TODO : discuss with Richard & Husseine
- Explain or give more information about the methods and how to choose the best parameters
ex : plv better with 100 samples & narrow bands
- Explain the choice either with ERP or without, which one to keep and why
- Explain Why to do not use sample_introduction data the for connectivity tuto.
- Show/add other relevant measures of statistics to separate the two conditions
- Add the option : checkbox remove the erp for PLV and CORR and PTE
- ...
TODO : Connectivity measure on MEG data
For all the brain imaging experiments, it is highly recommended to have a clear hypothesis to test before starting the analysis of the recordings. With this auditory oddball experiment, we would like to explore the temporal dynamics of the auditory network, the deviant detection and the motor response. According to the literature, we expect to observe at least the following effects:
Bilateral response in the primary auditory cortex (P50, N100), in both experimental conditions (standard and deviant beeps).
Bilateral activity in the inferior frontal gyrus and the auditory cortex corresponding to the detection of an abnormality (latency: 150-250ms) for the deviant beeps only.
Decision making and motor preparation, for the deviant beeps only (after 300ms).
TODO : Electrodes level
TODO : Sources level
We can compute the connectivity matrix between all pairs of dipoles. This process is possibel only of the inverse problem is computed (ref to tuto here). In the process tab you need to drag and drop the source file within the process1 tab, select the option 'source process' click on the Run button. You can select the connectivity measure that you want to perform.
As in previous section, we can compute the source connectivity matrix for each trail, then average over all trial. However this process is time and memory consuming. For each trail a matrix of 15002x15002 elements is computed and saved in the hard disc (~0.9 Go per trial). In the case of the unconstrained source, the size is 45006x45006.
This is obviously a very large number and it does not really make sense. Therefore, the strategy is to reduce the dimensionality of the source space and adopt a parcellation scheme, in other terms we will use the scouts. Unstead to compute the connectivity value values between two dipoles, we will use a sets of dipoles pairs that belong to a given area in the cortex.
Although the choice of the optimal parcellation scheme for the source space is not easy. The optimal choice is to choose a parcellation based on anatomy, for example the Brodmann parcellation here. In brainstorm these atlases are imported in Brainstorm as scouts (cortical regions of interest), and saved directly in the surface files as explained in this tutorial here.
In this tutorial, we will use the scouts " Destrieux atlas" (following figure) ![]()
To select this atlas, from the connectivity menu, you have to check the box 'use scouts', select the scout function 'mean' and apply the function 'Before', save individual results.
In this tutorial, we select the correlation as example, the same process is expected for the other methods.
For more detail for these options please refer to this thread
The following matrix is the solution that we obtain with these scouts with the size of 148x148 for this atlas (~400 Ko)
Granger Causality - Mathematical Background
Practical issues about GC:
Temporal resolution: the high time resolution offered by MEG/EEG and intracranial EEG allows for a very powerful application of GC and also offers the important advantage of spectral analysis.
Stationarity: the GC methods described so far are all based on AR models, and therefore assume stationarity of the signal (constant auto-correlation over time). However, neuroscience data, especially task-based data such as event-related potentials are mostly nonstationary. There are two possible approaches to solve this problem. The first is to apply methods such as differencing, filtering, and smoothing to make the data stationary (see a recommendation for time domain GC). Dynamical changes in the connectivity profile cannot be detected with the first approach. The second approach is to turn to versions of GC that have been adapted for nonstationary data, either by using a non-parametric estimation of GC or through measures of time-varying GC, which estimate dynamic parameters with adaptive or short-time window methods (Bressler and Seth, 2011).
Number of variables: Granger causality is very time-consuming in the multivariate case for many variables (O(m^2) where m represents the number of variables). Since each connection pair results in two values, there will also be a large number of statistical comparisons that need to be controlled for. When performing GC in the spectral domain, this number increases even more as statistical tests have to be performed per frequency. Therefore, it is usually recommended to select a limited number of ROIs or electrodes based on some hypothesis found in previous literature, or on some initial processing with a more simple and less computationally heavy measure of connectivity.
Pre-processing: The influence of pre-processing steps such as filtering and smoothing on GC estimates is a crucial issue. Studies have generally suggested to limit filtering only for artifact removal or to improve the stationarity of the data but cautioned against band-pass filtering to isolate causal influence within a specific frequency band (Barnett and Seth, 2011).
Volume Conduction: Granger causality can be performed both in the scalp domain or in the source domain. Though spectral domain GC generally does not incorporate present values of the signals in the model, it is still not immune from spurious connectivity measures due to volume conduction (for a discussion see (Steen et al., 2016)). Therefore, it is recommended to reduce the problem of signal mixing using additional processing steps such as performing source localization and doing connectivity in the source domain.
Data length: because of the extent of parameters that need to be estimated, the number of data points should be sufficient for a good fit of the model. This is especially true for windowing approaches, where data is cut into smaller epochs. A rule of thumb is that the number of estimated parameters should be at least (~10) several times smaller than the number of data points.
Additional documentation
References
Articles
Phase transfer entropy: Lobier M, Siebenhühner F, Palva S, Palva JM Phase transfer entropy: A novel phase-based measure for directed connectivity in networks coupled by oscillatory interactions, NeuroImage 2014, 85:853-872
Forum discussions
Connectivity matrix storage:http://neuroimage.usc.edu/forums/showthread.php?1796
Comparing coherence values: http://neuroimage.usc.edu/forums/showthread.php?1556
Reading NxN PLV matrix: http://neuroimage.usc.edu/forums/t/pte-how-is-the-connectivity-matrix-stored/4618/2
Scout function and connectivity: http://neuroimage.usc.edu/forums/showthread.php?2843
Unconstrained sources and connectivity: http://neuroimage.usc.edu/forums/t/problem-with-surfaces-vs-volumes/3261
Digonal values: http://neuroimage.usc.edu/forums/t/choosing-scout-function-before-or-after/2454/2
Connectivity pipeline: https://neuroimage.usc.edu/forums/t/help-for-connectivity-pipeline/12558/4
Ongoing developments: https://neuroimage.usc.edu/forums/t/connectivity-tutorial-and-methods-development-on-bst/12223/3
Granger causality: https://neuroimage.usc.edu/forums/t/is-granger-causality-analysis-a-linear-operation/12506/12
