|
Size: 8821
Comment:
|
Size: 14284
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 8: | Line 8: |
| Requirements: You need a license for the Matlab environment in order to use these tools and execute custom scripts. If you are running the compiled version of Brainstorm with the MCR library, the only custom code you can run is through the menu File > Matlab console and the process "Run Matlab command". |
|
| Line 9: | Line 11: |
== Starting a new script == The easiest way to get started with a new Brainstorm script is to use the script generator, already introduced in the tutorial [[http://neuroimage.usc.edu/brainstorm/Tutorials/PipelineEditor#Saving_a_pipeline|Select files and run processes]]. Select some files in the Process1 or Process2 tabs, select a list of processes, and use the menu '''Generate .m script'''. The example below should work on the the protocol "TutorialIntroduction" created during the introduction tutorials. * In the Process1 tab, leave the selection box empty and click on [Run]. Instead of selecting the files from the Brainstorm interface, we will select them directly from the database using a script. * Select process '''File > Select files: Recordings''' (do not execute immediately)<<BR>>Subject='''Subject01''', Condition='''[Empty]''', File comment='''Avg: deviant''' (the space is important). <<BR>><<BR>> {{attachment:start1.gif}} * This process selects all the recordings with a comment including the string "Avg: deviant", from all the folders in Subject01 (except "Intra-subject" and "Common files"). We expect to get two files: the averages of the deviant condition for both runs. * Add process '''Pre-process > Band-pass filter''': Lower cutoff='''0Hz''', Upper cutoff='''30Hz''', Mirror. <<BR>> '''Add process File > Save snapshot''': Recordings time series, Sensors=MEG. <<BR>> <<BR>> {{attachment:start2.gif}} * This will apply a low-pass filter at 30Hz and save a screen capture of the signals in the report. * Do not run the pipeline, select the menu '''Generate .m script '''instead. It saves a new .m file and opens it in the Matlab editor. Close the pipeline editor window and look at the script.<<BR>><<BR>> {{attachment:start3.gif}} == Anatomy of a generated script == The script you just generated can be the starting point to your own custom script. The following sections explain how they work and how to edit them. === Script header === === Script body === You will find one block per process you selected. They all have the same syntax: <<BR>> output_files = '''bst_process'''('CallProcess', process_name, input_files_A, input_files_B, options_list); * '''process_name''': String indicating the function corresponding to the process to execute. To know from the pipeline editor what is the path to the process function: hover your mouse over the selected process, as illustrated in [[http://neuroimage.usc.edu/brainstorm/Tutorials/PipelineEditor#Saving_a_pipeline|this tutorial]]. * '''input_files_A''': List of input files in Process1, or FilesA in Process2. It can be a cell array of files names (full path, or relative path from the protocol folder), or an array of structures describing the files in the database (returned by a previous call to bst_process). * '''input_files_B''': Empty for Process1, or FilesB in Process2. Cell array of strings or array of struct. * '''options_list''': Pairs of (option_name, option_values), one for each option of the process. * '''output_files''': Array of structures describing the files in output of the process. If the process created new files, this variable contains the new files. If the process didn't create new files or was modifying exiting files, most of the time this variable would contain the same files as the input list. {{{ % Process: Select data files in: Subject01/*/Avg: deviant sFiles = bst_process('CallProcess', 'process_select_files_data', sFiles, [], ... 'subjectname', SubjectNames{1}, ... 'condition', '', ... 'tag', 'Avg: deviant', ... 'includebad', 0, ... 'includeintra', 0, ... 'includecommon', 0); % Process: Low-pass:30Hz sFiles = bst_process('CallProcess', 'process_bandpass', sFiles, [], ... 'highpass', 0, ... 'lowpass', 30, ... 'mirror', 1, ... 'sensortypes', 'MEG, EEG', ... 'overwrite', 0); % Process: Snapshot: Recordings time series sFiles = bst_process('CallProcess', 'process_snapshot', sFiles, [], ... 'target', 5, ... % Recordings time series 'modality', 1, ... % MEG (All) 'orient', 4, ... % bottom 'time', 0.11, ... 'contact_time', [0, 0.1], ... 'contact_nimage', 12, ... 'threshold', 20, ... 'Comment', 'Run'); }}} === Script footer === == Editing the script == You can edit this section manually, particularly to edit the options or change the input/outputs. The options are easy to read and understand: == Starting Brainstorm == . - gui / nogui - selecting protocol - delete existing protocol == Selecting files == - Inputs / outputs - Select processes - Adding tags to help with the file selection later == Database requests == == File manipulation == * Modify a structure manually: Export to Matlab/Import from Matlab * File manipulation: file_short, file_fullpath, in_bst_*... * Documentation of all file structures: point at the appropriate tutorials * Select files from the database (with bst_get and processes) == Loop over subject and runs == Creating loops is not supported yet by the script generator, but relatively easy to do from a script without having to know too much about Matlab programming. 1) Fill the cell array SubjectNames with all your subjects names, with the same dimensions as the list of input raw files (sFiles) . 2) Add a "for" loop that includes all the bst_process() calls (leave the bst_report() calls and input definition outside) 3) Inside the loop, replace SubjectNames with SubjectNames{i} and sFiles with sFiles(i) |
|
| Line 56: | Line 139: |
| == Script generation == http://neuroimage.usc.edu/brainstorm/Tutorials/PipelineEditor == Script edition == Add loops, load files, ... Loops: http://neuroimage.usc.edu/forums/showthread.php?2429-Problem-using-tags == File manipulation == * Modify a structure manually: Export to Matlab/Import from Matlab * File manipulation: file_short, file_fullpath, in_bst_*... * Documentation of all file structures: point at the appropriate tutorials * Select files from the database (with bst_get and processes) |
|
| Line 71: | Line 140: |
| The following script from the Brainstorm distribution reproduces all the introduction tutorials: '''brainstorm3/toolbox/script/tutorial_introduction.m''' | The following script from the Brainstorm distribution reproduces the introduction tutorials ("Get started"): '''brainstorm3/toolbox/script/tutorial_introduction.m''' |
| Line 75: | Line 144: |
| <<BR>><<BR>>For an example of a script illustrating how to create loops, look at the tutorial MEG visual: group study: '''brainstorm3/toolbox/script/tutorial_visual_''''''group.m''' | <<BR>>For an example of a script illustrating how to create loops, look at the tutorial [[Tutorials/VisualSingle|MEG visual: single subject]]. '''brainstorm3/toolbox/script/tutorial_visual_single.m''' |
| Line 77: | Line 146: |
| <<HTML(<div style="border:1px solid black; background-color:#EEEEFF; width:720px; height:500px; overflow:scroll; padding:10px; font-family: Consolas,Menlo,Monaco,Lucida Console,Liberation Mono,DejaVu Sans Mono,Bitstream Vera Sans Mono,Courier New,monospace,sans-serif; font-size: 13px; white-space: pre;">)>><<EmbedContent("http://neuroimage.usc.edu/bst/viewcode.php?f=tutorial_visual_group.m")>><<HTML(</div >)>> | <<HTML(<div style="border:1px solid black; background-color:#EEEEFF; width:720px; height:500px; overflow:scroll; padding:10px; font-family: Consolas,Menlo,Monaco,Lucida Console,Liberation Mono,DejaVu Sans Mono,Bitstream Vera Sans Mono,Courier New,monospace,sans-serif; font-size: 13px; white-space: pre;">)>><<EmbedContent("http://neuroimage.usc.edu/bst/viewcode.php?f=tutorial_visual_single.m")>><<HTML(</div >)>> |
Tutorial 28: Scripting
[TUTORIAL UNDER DEVELOPMENT: NOT READY FOR PUBLIC USE]
Authors: Francois Tadel, Elizabeth Bock, Sylvain Baillet
The previous tutorials explained how to use Brainstorm in an interactive way to process one subject with two acquisition runs. In the context of a typical neuroimaging study, you may have tens or hundreds of subjects to process in the same way, it is unrealistic to do everything manually. Some parts of the analysis can be processed in batches with no direct supervision, others require more attention. This tutorial introduces tools and tricks that will help you assemble an efficient analysis pipeline.
Requirements: You need a license for the Matlab environment in order to use these tools and execute custom scripts. If you are running the compiled version of Brainstorm with the MCR library, the only custom code you can run is through the menu File > Matlab console and the process "Run Matlab command".
Contents
Starting a new script
The easiest way to get started with a new Brainstorm script is to use the script generator, already introduced in the tutorial Select files and run processes. Select some files in the Process1 or Process2 tabs, select a list of processes, and use the menu Generate .m script. The example below should work on the the protocol "TutorialIntroduction" created during the introduction tutorials.
- In the Process1 tab, leave the selection box empty and click on [Run]. Instead of selecting the files from the Brainstorm interface, we will select them directly from the database using a script.
Select process File > Select files: Recordings (do not execute immediately)
Subject=Subject01, Condition=[Empty], File comment=Avg: deviant (the space is important).
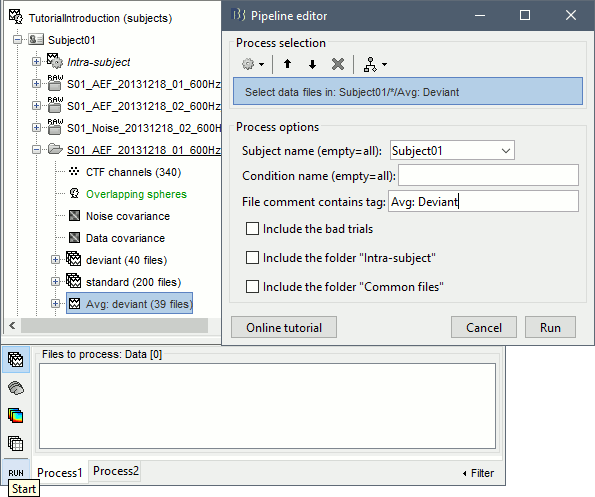
- This process selects all the recordings with a comment including the string "Avg: deviant", from all the folders in Subject01 (except "Intra-subject" and "Common files"). We expect to get two files: the averages of the deviant condition for both runs.
Add process Pre-process > Band-pass filter: Lower cutoff=0Hz, Upper cutoff=30Hz, Mirror.
Add process File > Save snapshot: Recordings time series, Sensors=MEG.
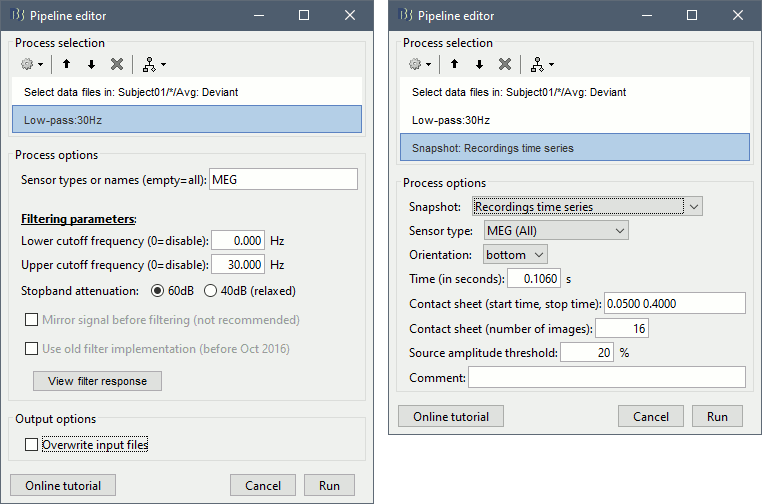
- This will apply a low-pass filter at 30Hz and save a screen capture of the signals in the report.
Do not run the pipeline, select the menu Generate .m script instead. It saves a new .m file and opens it in the Matlab editor. Close the pipeline editor window and look at the script.
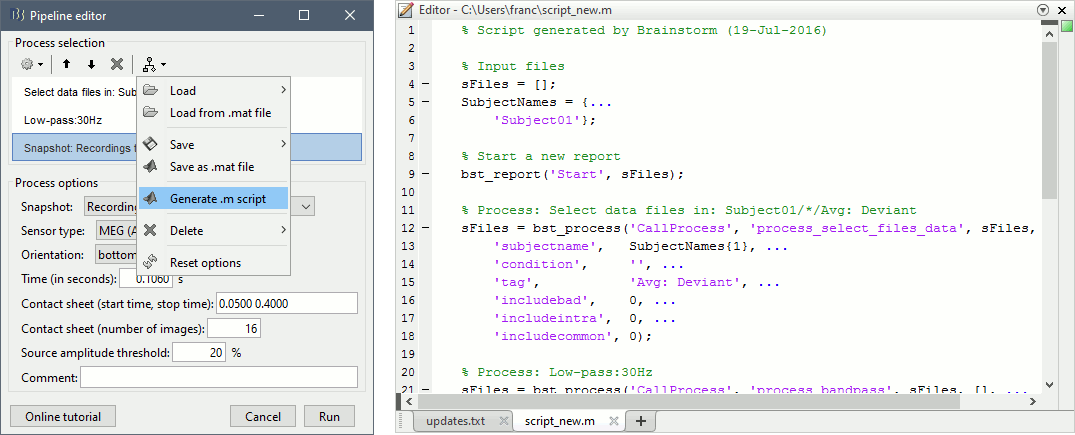
Anatomy of a generated script
The script you just generated can be the starting point to your own custom script. The following sections explain how they work and how to edit them.
Script header
Script body
You will find one block per process you selected. They all have the same syntax:
output_files = bst_process('CallProcess', process_name, input_files_A, input_files_B, options_list);
process_name: String indicating the function corresponding to the process to execute. To know from the pipeline editor what is the path to the process function: hover your mouse over the selected process, as illustrated in this tutorial.
input_files_A: List of input files in Process1, or FilesA in Process2. It can be a cell array of files names (full path, or relative path from the protocol folder), or an array of structures describing the files in the database (returned by a previous call to bst_process).
input_files_B: Empty for Process1, or FilesB in Process2. Cell array of strings or array of struct.
options_list: Pairs of (option_name, option_values), one for each option of the process.
output_files: Array of structures describing the files in output of the process. If the process created new files, this variable contains the new files. If the process didn't create new files or was modifying exiting files, most of the time this variable would contain the same files as the input list.
% Process: Select data files in: Subject01/*/Avg: deviant
sFiles = bst_process('CallProcess', 'process_select_files_data', sFiles, [], ...
'subjectname', SubjectNames{1}, ...
'condition', '', ...
'tag', 'Avg: deviant', ...
'includebad', 0, ...
'includeintra', 0, ...
'includecommon', 0);
% Process: Low-pass:30Hz
sFiles = bst_process('CallProcess', 'process_bandpass', sFiles, [], ...
'highpass', 0, ...
'lowpass', 30, ...
'mirror', 1, ...
'sensortypes', 'MEG, EEG', ...
'overwrite', 0);
% Process: Snapshot: Recordings time series
sFiles = bst_process('CallProcess', 'process_snapshot', sFiles, [], ...
'target', 5, ... % Recordings time series
'modality', 1, ... % MEG (All)
'orient', 4, ... % bottom
'time', 0.11, ...
'contact_time', [0, 0.1], ...
'contact_nimage', 12, ...
'threshold', 20, ...
'Comment', 'Run');
Script footer
Editing the script
You can edit this section manually, particularly to edit the options or change the input/outputs. The options are easy to read and understand:
Starting Brainstorm
- - gui / nogui - selecting protocol - delete existing protocol
Selecting files
- Inputs / outputs
- Select processes
- Adding tags to help with the file selection later
Database requests
File manipulation
- Modify a structure manually: Export to Matlab/Import from Matlab
- File manipulation: file_short, file_fullpath, in_bst_*...
- Documentation of all file structures: point at the appropriate tutorials
- Select files from the database (with bst_get and processes)
Loop over subject and runs
Creating loops is not supported yet by the script generator, but relatively easy to do from a script without having to know too much about Matlab programming.
1) Fill the cell array SubjectNames with all your subjects names, with the same dimensions as the list of input raw files (sFiles)
- 2) Add a "for" loop that includes all the bst_process() calls (leave the bst_report() calls and input definition outside)
3) Inside the loop, replace SubjectNames with SubjectNames{i} and sFiles with sFiles(i)
How to process many subjects
This section proposes a standard workflow for processing a full group study with Brainstorm. It contains the same steps of analysis as the introduction tutorials, but separating what can be done automatically from what should be done manually. This workflow can be adapted to most ERP studies (stimulus-based).
Prototype: Start by processing one or two subjects completely interactively (exactly like in the introduction tutorials). Use the few pilot subjects that you have for your study to prototype the analysis pipeline and check manually all the intermediate stages. Take notes of what you're doing along the way, so that you can later write a script that reproduces the same operations.
Anatomical fiducials: Set NAS/LPA/RPA and compute the MNI transformation for each subject.
Segmentation: Run FreeSurfer/BrainSuite to get surfaces and atlases for all the subjects.
File > Batch MRI fiducials: This menu prompts for the selection of the fiducials for all the subjects and saves a file fiducials.m in each segmentation folder. You will not have to redo this even if you have to start over your analysis from the beginning.
Script: Write a loop that calls the process "Import anatomy folder" for all the subjects.
Alternatives: Create and import the subjects one by one and set the fiducials at the import time. Or use the default anatomy for all the subjects (or use warped templates).
Script #1: Pre-processing: Loop on the subjects and the acquisition runs.
Create link to raw files: Link the subject and noise recordings to the database.
Event markers: Read and group triggers from digital and analog channel, fix stimulation delays
Evaluation: Power spectrum density of the recordings to evaluate their quality.
Pre-processing: Notch filter, sinusoid removal, band-pass filter.
Evaluation: Power spectrum density of the recordings to make sure the filters worked well.
Cleanup: Delete the links to the original files (the filtered ones are copied in the database).
Detect artifacts: Detect heartbeats, Detect eye blinks, Remove simultaneous.
Compute SSP: Heartbeats, Blinks (this selects the first component of each decomposition)
Compute ICA: If you have some artifacts you'd like to remove with ICA (no default selection).
Screenshots: Check the MRI/sensors registration, PSD before and after corrections, SSP.
Export the report: One report per subject, or one report for all the subjects, saved in HTML.
Manual inspection #1:
Check the reports: Information messages (number of events, errors and warnings) and screen captures (registration problems, obvious noisy channels, incorrect SSP topographies).
Mark bad channels: Open the recordings, select the channels and mark them as bad. Or use the process "Set bad channels" to mark the same bad channels in multiple files.
Fix the SSP/ICA: For the suspicious runs: Open the file, adjust the list of blink and cardiac events, remove and recompute the SSP decompositions, manually select the components.
Detect other artifacts: Run the process on all the runs of all the subjects at once (select all the files in Process1 and run the process, or generate the equivalent script).
Mark bad segments: Review the artifacts detected in 1-7Hz and 40-240Hz, keep only the ones you really want to remove, then mark the event categories as bad. Review quickly the rest of the file and check that there are no other important artifacts.
Additional SSP: If you find one type of artifact that repeats (typically saccades and SQUID jumps), you can create additional SSP projectors, either with the process "SSP: Generic" or directly from a topography figure (right-click on the figure > Snapshot> Use as SSP projector).
Script #2: Subject-level analysis: Epoching, averaging, sources, time-frequency.
Importing: Process "Import MEG/EEG: Events" and "Pre-process > Remove DC offset".
Averaging: Average trials by run, average runs by subject (registration problem in MEG).
Noise covariance: Compute from empty room or resting recordings, copy to other folders.
Head model: Compute for each run, or compute once and copy if the runs are co-registered.
Sources: Compute for each run, average across runs and subjects in source space for MEG.
Time-frequency: Computation with Hilbert transform or Morlet wavelets, then normalize.
Screenshots: Check the quality of all the averages (time series, topographies, sources).
Export the report: One report per subject, or one report for all the subjects, saved in HTML.
Manual inspection #2:
Check the reports: Check the number of epochs imported and averaged in each condition, check the screen capture of the averages (all the primary responses should be clearly visible).
Regions of interest: If not using predefined regions from an atlas, define the scouts on the anatomy of each subject (or on the template and then project them to the subjects).
Script #3: Group analysis, ROI-based analysis, etc.
Averaging: Group averages for the sensor data, the sources and the time-frequency maps.
Statistics: Contrast between conditions or groups of subjects.
Regions of interest: Any operation that involve scouts.
Final script
The following script from the Brainstorm distribution reproduces the introduction tutorials ("Get started"): brainstorm3/toolbox/script/tutorial_introduction.m
For an example of a script illustrating how to create loops, look at the tutorial MEG visual: single subject. brainstorm3/toolbox/script/tutorial_visual_single.m
Report viewer
Click on Run to start the script.
As this process is taking screen captures, do not use your computer for something else at the same time: if another window covers the Brainstorm figures, it will not capture the right images.
At the end, the report viewer is opened to show the status of all the processes, the information messages, the list of input and output files, and the screen captures. The report is saved in your home folder ($home/.brainstorm/reports). If you close this window, you can get it back with the menu File > Report viewer.
